Stem Cell Res. 2020 May;45:101770
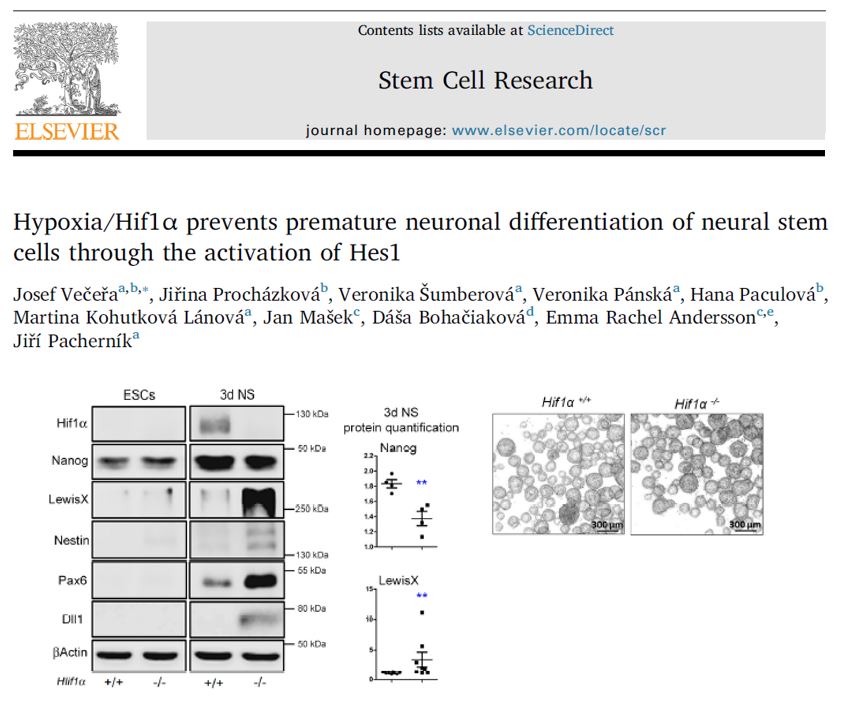
ABSTRACT: Embryonic neural stem cells (NSCs), comprising neuroepithelial and radial glial cells, are indispensable precursors of neurons and glia in the mammalian developing brain. Since the process of neurogenesis occurs in a hypoxic environment, the question arises of how NSCs deal with low oxygen tension and whether it affects their stemness. Genes from the hypoxia-inducible factors (HIF) family are well known factors governing cellular response to hypoxic conditions. In this study, we have discovered that the endogenous stabilization of hypoxia-inducible factor 1α (Hif1α) during neural induction is critical for the normal development of the NSCs pool by preventing its premature depletion and differentiation. The knock-out of the Hif1α gene in mESC-derived neurospheres led to a decrease in self-renewal of NSCs, paralleled by an increase in neuronal differentiation. Similarly, neuroepithelial cells differentiated in hypoxia exhibited accelerated neurogenesis soon after Hif1α knock-down. In both models, the loss of Hif1α was accompanied by an immediate drop in neural repressor Hes1 levels while changes in Notch signaling were not observed. We found that active Hif1α/Arnt1 transcription complex bound to the evolutionarily conserved site in Hes1 gene promoter in both neuroepithelial cells and neural tissue of E8.5 – 9.5 embryos. Taken together, these results emphasize the novel role of Hif1α in the regulation of early NSCs population through the activation of neural repressor Hes1, independently of Notch signaling.