——–
What do we work with in our laboratory? We work with early stages of Xenopus frog embryos. 🐸
Why frogs? As you may have noticed in nature, frog embryos are generally relatively large and develop outside the mother’s body, making them excellent for observation under a microscope. 🔬
Why Xenopus and not another frog? Xenopus was used as the first reliable test for fertility in humans. It was called the Hogben test, named after the British scientist Lancelot Hogben, and was used in American and English hospitals from the 1930s until World War II. After biochemical strips replaced these tests, Xenopus frogs became the subject of study in hospitals and scientific centers, becoming the most commonly used amphibian or frog models in biomedical research. 🧪
What do we study in Xenopus frog embryos? We study the formation of the neural tube and the migration of cells in the so-called neural crest. 🧠
—————-
Want to discover more? Click the link to our short reportages on Czech Radio or Czech TV broadcasts (both in Czech), and the popular biology blog, The Node (in English).
————-
PRINCIPAL INVESTIGATOR

Group leader: Assist. Prof. Jakub Harnos, Ph.D.
Office: Campus Bohunice, bldg. D36, rm 1S16
E-mail: harnos@sci.muni.cz
Telephone: +420 549 49 4465
ORCID ID: 0000-0002-0752-9260
————-
Scientific autobiography
Throughout my graduate training, I have focused on cellular pathways, particularly WNT signaling, vital for multicellular development and implicated in various diseases when dysregulated. Under the supervision of Prof. Vitezslav Bryja, I discovered a new functional relationship between CK1 kinases, Dishevelled, and Axin, the central components of WNT signaling (Harnos et al. 2018). Collaborating with Prof. Carsten Hoffman at the University of Wurzburg, I developed FRET biosensors to monitor the dynamics of Dishevelled’s structural conformations and to describe their specific roles in WNT signal transduction (Harnos et al. 2019).
During my postdoctoral work with Prof. Sergei Sokol in New York, I deepened my expertise in vertebrate embryology and the Xenopus model system, focusing on non-canonical WNT/PCP (planar cell polarity) signaling.
I explored PCP-interacting proteins during neurulation in Xenopus embryos using proteomics and microscopy approaches.
Upon returning to Czechia in the Summer of 2020, I took on the role of Assistant Professor at MUNI.
I successfully established my own lab, secured funding, and published several research papers as an independent PI, which marked a significant step toward scientific independence. My research now spans three main areas: cellular signaling in vertebrates, vertebrate development, and in-cell nucleic acid structure. These diverse topics are unified by the use of the Xenopus model (i.e., Xenopus oocytes, embryos, tadpoles, and adult frogs), which remains central to my research group’s work.
This interdisciplinary approach aims to uncover novel mechanisms and potentially improve disease treatments driven by cell migration and neurulation.
Fellowships, Grants, and Awards
*2025 – 2026: MSCAfellow5_MUNI (#22_010/0003229), Czech MEYS, Czechia
*2024 – 2026: Grant in the Standard/Senior category (#24-10622S), Czech Science Foundation, Czechia
2023: Hemsley Fellowship (#203103), Xenopus course, Cold Spring Harbor Laboratories, NY, USA (declined due to family reasons)
2022 – 2024: Grant in the Standard/Senior category (#22-06405S), Czech Science Foundation, Czechia
2022 – 2024: Science & Humanities Award Junior (#MUNI/J/0004/2021), Grant Agency of Masaryk University, Czechia
2020: “Seal of Excellence” in MSCA-IF-2020 (#101028952), European Commission, Brussels
2018 – 2019: “Excellent Results” Grant (#MUNI/E/0533/2018), Grant Agency of Masaryk University, Czechia
2017 – 2017: EMBO Short-term Fellowship (#ASTF 687-2016), University of Wurzburg, Germany
2016 – 2016: Mobility program “Free mover”, University of Wurzburg, Germany
*Current funding
————-
RESEARCH TOPIC
Polarity refers to spatial differences in shape, structure, and function within a cell. Almost all cell types exhibit some form of polarity that enables them to carry out specialized functions. We focus on planar polarity, which refers to the coordinated alignment of cells across the tissue plane. Planar polarity is currently viewed as a “passive” compass providing cells with a sense of direction. This feature is important during development when a cell needs to know its position within a multicellular organism, and during homeostasis, when a cell needs to know the direction e.g., for its migration.
Our primary goal is to demonstrate the active role of planar polarity in neural tube formation during vertebrate development. Neural tube formation is an early developmental event that involves approximately two hundred proteins. Although the tube formation is described somewhat well, the factor responsible for its initiation is not yet known. We have gathered evidence that polarity proteins may be the active triggers for initiating neural tube formation.
Our second aim is related to cell migration. Migration is the directed movement of a cell from one place to another and requires an increased amount of energy. The produced energy is utilized to rearrange dedicated regions in a migratory cell, thus allowing its physical movement. However, what initiates the production of the extra energy needed for rearrangements remains a mystery. Here, we intend to show the active role of planar polarity as an energy trigger for cell migration.
In short, assigning dynamic behaviors to polarity proteins in Xenopus embryos and tissue culture systems is the core of the research conducted in the Harnos lab.
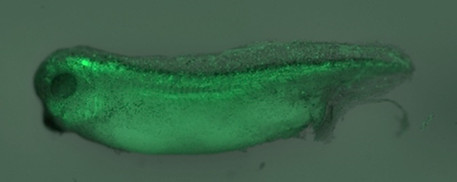
LEFT: A developing Xenopus tadpole, whose neural cells have been marked with the green fluorescent protein (GFP).
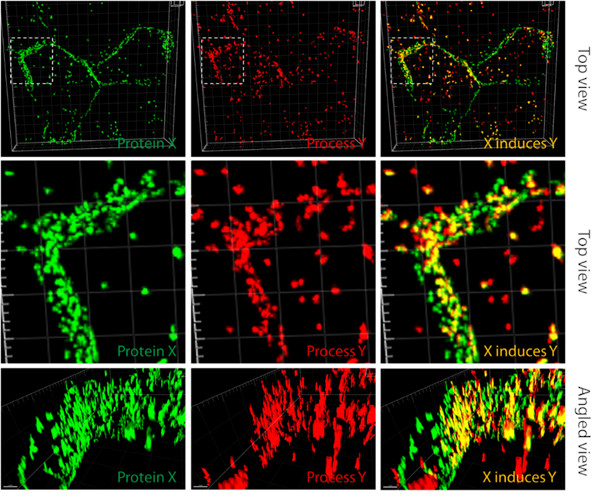
RIGHT: We investigate cellular processes in which proteins can initiate several subsequent events that we can observe using microscopes.
In the picture, there are two cells expressing green fluorescent protein fused to a protein of our interest. The last picture on the right shows that the green fusion protein co-localizes with the red cellular process, suggesting that protein X induces this particular cellular process Y (images were edited in Imaris software v9.8).
Keywords
Planar polarity, neural tube formation, cell migration, bioenergetics, tissue culture cells, Xenopus embryos.
SCIENTIFIC PAPERS
- Cellular signaling in vertebrates (planar cell polarity signaling and cilia formation):
- Radaszkiewicz KA, Sulcova M, Kohoutkova E, and Harnos J* (*corresponding author). “The role of Prickle proteins in vertebrate development and pathogeny.” Molecular Cell Biochemistry, 2023, doi: 10.1007/s11010-023-04787-z. This research investigates how Prickle proteins impact vertebrate development and their involvement in disease, shedding light
on the molecular mechanisms of planar cell polarity.
- Novotna S, Maia LA, Radaszkiewicz KA, Roudnicky P, and Harnos J* (*corresponding author). “Linking planar polarity signaling to actomyosin contractility during vertebrate neurulation.” Open Biology, 2024, doi: 10.1098/rsob.240251. This recent study explores the molecular relationship between planar polarity signaling and actomyosin contractility during vertebrate neurulation in Xenopus, providing insights into tissue morphogenesis.
- Kravec M, Sedo O, Nedvedova J, Micka M,…, Harnos J, Tripsianes K, Janke C, Barinka C, and Bryja V. “Carboxy-terminal polyglutamylation regulates signalling and phase separation of the Dishevelled protein.” EMBO Journal, 2024, doi: 10.1038/s44318-024-00254-7. This study reveals a new mechanism that impacts the signalling and phase separation of the Dishevelled protein, providing new insights into WNT pathway regulation, including Xenopus embryos.
- Radaszkiewicz KA, Sulcova M, Kohoutkova E, and Harnos J* (*corresponding author). “The role of Prickle proteins in vertebrate development and pathogeny.” Molecular Cell Biochemistry, 2023, doi: 10.1007/s11010-023-04787-z. This research investigates how Prickle proteins impact vertebrate development and their involvement in disease, shedding light
- New aspects of vertebrate development:
- Laznovsky J, Kavkova M, Reis A, Robovska-Havelkova P, Maia LA, Krivanek J, Zikmund T, Kaiser J, Buchtova M, and Harnos J* (*corresponding author). “Unveiling vertebrate development dynamics in Xenopus laevis using micro-CT imaging.” GigaScience, 2024, doi: 10.1093/gigascience/giae037. This paper introduces advanced micro-CT imaging techniques that provide new insights into vertebrate development and both soft and hard tissue formation in Xenopus laevis, focusing on skeletal, brain, and gut development.
- Gonzalez Lopez M, Huteckova B, Lavicky J, Zezula N,…, Harnos J, Buchtova M, and Krivanek J. “Spatiotemporal monitoring of hard tissue development reveals new features of tooth and bone development.” Science Advances, 2023, doi: 10.1126/sciadv.adi0482. This research uses spatiotemporal monitoring to reveal novel aspects of tooth and bone development, enhancing our understanding of hard tissue formation in key vertebrate models, including Xenopus.
- Studying in-cell DNA quadruplex structures using Xenopus oocytes:
- Foldynova-Trantirkova S, Harnos J (co-shared first author), Rynes J, Zlinska V, and Trantirek L. “In-cell NMR spectroscopy of nucleic acids: Basic concepts, practical aspects, and applications.” Progress in Nuclear Magnetic Resonance Spectroscopy, 2025, doi: 10.1016/j.pnmrs.2025.101560. This invited review summarizes recent advances in the use of in-cell NMR spectroscopy to study nucleic acids, with a focus on their structure and function in an in vivo environment.
I contributed by discussing the biological aspects, including Xenopus oocytes.
- Foldynova-Trantirkova S, Harnos J (co-shared first author), Rynes J, Zlinska V, and Trantirek L. “In-cell NMR spectroscopy of nucleic acids: Basic concepts, practical aspects, and applications.” Progress in Nuclear Magnetic Resonance Spectroscopy, 2025, doi: 10.1016/j.pnmrs.2025.101560. This invited review summarizes recent advances in the use of in-cell NMR spectroscopy to study nucleic acids, with a focus on their structure and function in an in vivo environment.
- Additionally, I recently developed a new biochemical approach: Paclikova P, and Harnos J* (*corresponding author). “Efficient cloning of linear DNA inserts (ECOLI) into plasmids using site-directed mutagenesis.” Scientific Reports, 2024, doi: 10.1038/s41598-024-72169-6. This study introduced a new cloning approach for DNA insert subcloning into plasmids that is cheap and efficient.
RESEARCH TEAM
- Postdoctoral fellows
- Katarzyna Radaszkiewicz, Ph.D., M.Sc. (currently on maternity leave)
- Lorena Agostini Maia, Ph.D.
- PhD students
- Lab technician
- Mgr. Julie Netusilova (currently on maternity leave)
- Dr. Douglas Porto Pereira Gomes
- Undergraduates
- Alumni
- Mgr. Petra Paclikova, Ph.D. (2024; currently a postdoc in Cajanek lab, MUNI, Czechia)
- Ing. Mgr. Vendula Janouskova (2023-2024; currently a Ph.D. student, Prague, Czechia)
- Mgr. Marie Sulcova, Ph.D. (2022-2023; currently a postdoc at the University of Stockholm, Sweden)
- Alba Hernández Ramos (2022-2023, Erasmus student, a practical 10-month stay, University of Salamanca, Spain)
- Mgr. Eliska Kohoutkova (2022-2023; currently a Ph.D. student at Vienna Biocenter, Austria)
- Cristina González Cuevas (2022, Erasmus student, a practical 3-month stay, University UFV in Madrid, Spain)
- Belén Escalona Pulido (2022, Erasmus student, a practical 4-month stay, University UFV in Madrid, Spain)
- Miriam Sánchez Calvo (2022, Erasmus student, a practical 4-month stay, University UFV in Madrid, Spain)



CONFERENCES (POSTER PRESENTATIONS)
- Armed with PRICKLE(3)s: PCP complexes protection against RNF43 RADASZKIEWICZ, Katarzyna Anna, Pavla KOLÁŘOVÁ, Tomasz Witold RADASZKIEWICZ, Petra PACLÍKOVÁ, Kristína GÖMÖRYOVÁ, Tomáš BÁRTA, Kateřina HANÁKOVÁ, Zbyněk ZDRÁHAL a Jakub HARNOŠ. Armed with PRICKLE(3)s: PCP complexes protection against RNF43. In Wnt Meeting 2024 – Heidelberg. 2024.
- Casein Kinase 1α fine-tunes NOTCH1 intracellular domain stability and function TURETTI, Fabio, Lorena AGOSTINI MAIA, Hana HAJŠMANOVÁ, Tomáš GYBEĽ, Marek DOKOUPIL, Vítězslav BRYJA, Jakub HARNOŠ a Jan MAŠEK. Casein Kinase 1α fine-tunes NOTCH1 intracellular domain stability and function. In Notch Signaling In Development, Regeneration and Disease (Gordon Research Conference ). 2024.
- Casein Kinase 1α fine-tunes NOTCH1 intracellular domain stability and function TURETTI, Fabio, Lorena AGOSTINI MAIA, Hana HAJŠMANOVÁ, Tomáš GYBEĽ, Marek DOKOUPIL, Vítězslav BRYJA, Jakub HARNOŠ a Jan MAŠEK. Casein Kinase 1α fine-tunes NOTCH1 intracellular domain stability and functions. In Wnt Meeting 2024 – Heidelberg. 2024.
- Linking planar polarity signaling to actomyosin contractility during vertebrate neurulation NOVOTNÁ, Šárka, Lorena AGOSTINI MAIA, Katarzyna Anna RADASZKIEWICZ, Pavel ROUDNICKÝ a Jakub HARNOŠ. Linking planar polarity signaling to actomyosin contractility during vertebrate neurulation. In Self-Organization in Biology: Freiburg Spemann-Mangold Centennial Symposium. 2024.
- Revealing the mechanism of the neural tube formation AGOSTINI MAIA, Lorena a Jakub HARNOŠ. Revealing the mechanism of the neural tube formation. In 2nd Conference of Signalling in Disease and Development. 2024.
- Revealing the mechanism of the neural tube formation AGOSTINI MAIA, Lorena a Jakub HARNOŠ. Revealing the mechanism of the neural tube formations. In Self-Organization in Biology: Freiburg Spemann-Mangold Centennial Symposium. 2024.
- Revealing the mechanism of the neural tube formation AGOSTINI MAIA, Lorena a Jakub HARNOŠ. Revealings the mechanism of the neural tube formation. In CSHL – Cell & Developmental Biology of Xenopus: Gene Discovery & Disease. 2024.
- The Role of PRICKLE3 in Cell Migration and Mitochondrial Metabolism: Implications for Cancer MetastasisKOLÁŘOVÁ, Pavla, Katarzyna Anna RADASZKIEWICZ, Aneta POUKOVÁ, Vendula JANOUŠKOVÁ a Jakub HARNOŠ. The Role of PRICKLE3 in Cell Migration and Mitochondrial Metabolism: Implications for Cancer Metastasis. In Mito Conference 2024. 2024.
- BEE-ST Method: 4D-Monitoring of biomineralization dynamics in bones and teeth. GONZÁLEZ LÓPEZ, Marcos, Barbora HUTEČKOVÁ, Josef LAVICKÝ, Nikodém ZEZULA, Vladislav RAKULTSEV, Vendula FRIDRICHOVÁ, Haneen Riadh Ali TUAIMA, Citta NOTTMEIER, Julian PETERSEN, Michaela KAVKOVÁ, Tomas ZIKMUND, Jozef KAISER, Rupali LAV, Haza STAR, Petr HENYS, Miroslav VORECHOVSKY, Vítězslav BRYJA, Abigail S. TUCKER, Jakub HARNOŠ, Marcela BUCHTOVÁ a Jan KŘIVÁNEK. BEE-ST Method: 4D-Monitoring of biomineralization dynamics in bones and teeth. In 3rd Conference of the Visegrad Group Society for Developmental Biology. 2023.
- Linking Wnt to Notch signaling using vertebrate embryos. ŠULCOVÁ, Marie, Fabio TURETTI, Marek DOKOUPIL, Jakub HARNOŠ a Jan MAŠEK. Linking Wnt to Notch signaling using vertebrate embryos. In 3rd Conference of the Visegrad Group Society for Developmental Biology. 2023.
- Understanding the Role of Casein Kinase 1α in the regulation of the Notch1 intracellular domain. ŠULCOVÁ, Marie, Hana HAJSMANOVA, Tomáš GYBEĽ, Vítězslav BRYJA, Jakub HARNOŠ a Jan MASEK. Understanding the Role of Casein Kinase 1α in the regulation of the Notch1 intracellular domain. In The Notch Meeting XII. 2023.
- Micro CT analysis of head development in Xenopus laevis. KAVKOVÁ, Michaela, Jakub HARNOŠ, Marie ŠULCOVÁ, Tomas ZIKMUND, Marcela BUCHTOVÁ a Josef KAISER. Micro CT analysis of head development in Xenopus laevis. In 19th International Congress of Developmental Biology. 2022.
- Seeking Help from Mitochondrial Biologists to Decipher the Role of New Mitochondrial Interactors. RADASZKIEWICZ, Katarzyna Anna, Aneta POUKOVÁ, Pavla KOLÁŘOVÁ a Jakub HARNOŠ. Seeking Help from Mitochondrial Biologists to Decipher the Role of New Mitochondrial Interactors. In Mitochondria in life, death and disease. 2022.
COLLABORATIONS
- Germany:
– Prof. Carsten Hoffmann (Maximilians University,Wurzburg/University Hospital, Jena)
– Prof. Alexandra Schambony (Max Planck Institute for Science of Light, Erlangen)
Previous collaborations led to my first-authored Nat Comm 2019 manuscript about Dishevelled
- USA:
– Prof. Sergei Sokol (Icahn School of Medicine at Mount Sinai, New York):
Active collaboration on neurulation and PCP signaling in developing Xenopus embryos
–
– Dr. Giovanna Collu (Icahn School of Medicine at Mount Sinai, New York):
Active collaboration in the context of WNT-Notch crosstalk
- Czechia:
– Assoc. Prof. Lukas Trantirek (CEITEC, Brno):
The previous collaboration led to my first-authored JBC 2018 manuscript about Axin
Active collaboration on DNA quadruplex structures analyzed by NMR techniques in Xenopus oocytes
– Prof. Zbynek Zdrahal (CEITEC, Brno):
The previous collaboration led to my first-authored Nat Comm 2019 manuscript about Dishevelled
Active collaboration on proximity biotinylation approaches using Mass Spectrometry
– Dr. Jan Masek (Charles University, Prague):
Active collaboration on cilia formation in Xenopus in the context of WNT-Notch crosstalk
We are actively recruiting talent of all levels, from Bachelor’s and Master’s students to PhDs and Postdocs.
Write an email with your CV and a short motivation letter to harnos@sci.muni.cz
Follow us on Twitter/X or BlueSky for the latest news and updates: